Introduction
To make, purify, and get peptides ready for use in pharmaceuticals, nutraceuticals, and diagnostics, peptide processing comprises complex industrial stages. These sequences of amino acids (2 to 50 residues) are now very useful for treating cancer, hormones, infections, and metabolic disorders.
By 2027, the worldwide peptide therapeutics industry is expected to be worth more than $60 billion. This is because of customised medicine, better drug selectivity, breakthroughs in synthesis, and more people recognising the benefits of peptide therapies. But processing peptides is hard in its way, and modern technologies like nanofiltration (NF) membranes, inline buffer dilution (ILBD), and Dynamic Axial Compression (DAC) chromatography are now overcoming these problems.
Challenges in Peptide Processing and Purification
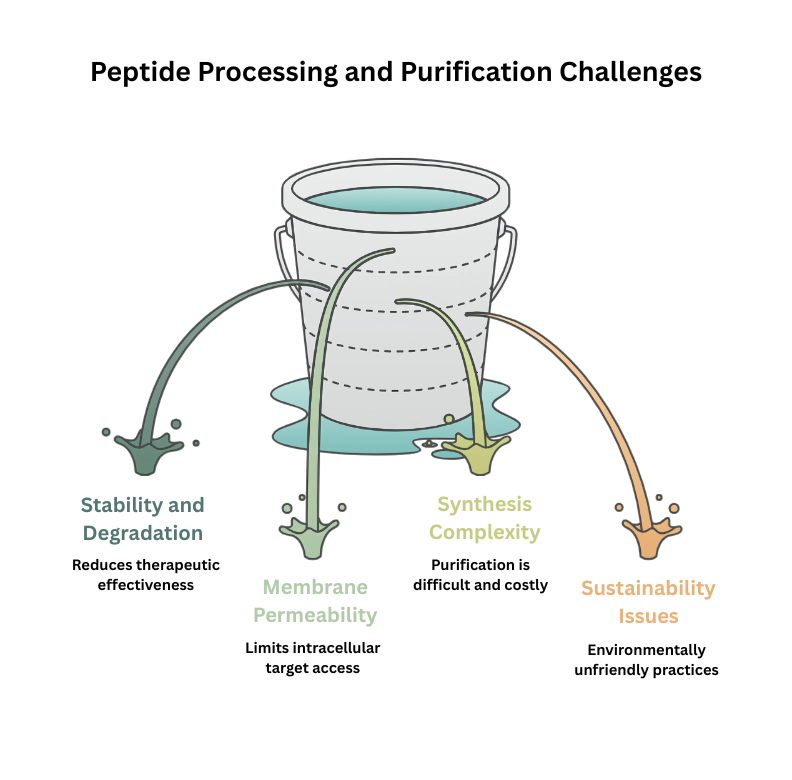
There are four big problems that peptide processing and purification have to deal with:
Problems with stability and degradation: Peptides become chemically fragile when amide bonds are hydrolysed, and enzymes quickly break them down through proteolysis. The effects of therapy are lessened, half-lives are short, and the quality and consistency of the product change from batch to batch.
Membrane Permeability: Peptides can’t get to their intracellular targets very well because they can’t get through the cell membrane. This makes them less available. This limitation makes it necessary to focus development on extracellular receptors, which limits the use of drugs and the ways they can be delivered.
Synthesis and Purification Complexity: Long peptide synthesis can have problems with imperfect reactions, aggregation, and the production of secondary structures. It is hard to purify because impurities have similar structures, which means that both specialist procedures and optimisation are needed.
Environmental and Economic Sustainability: Using a lot of dangerous organic solvents and chemicals in traditional peptide processing is bad for the environment. The industry is being pushed to use greener synthesis processes and more environmentally friendly practices because of high production costs, problems with scaling up, and the need to reduce waste.
Transformative Technologies
Nanofiltration (NF) Membrane Systems
Nanofiltration technique uses semi-permeable membranes with pore diameters that may be very carefully tuned and range from 1 to 10 nanometers. This placement between ultrafiltration and reverse osmosis lets NF systems separate molecules with amazing accuracy and speed.
NF membrane systems are great for peptide processing since they:
- Remove salts, organic solvents, and tiny contaminant compounds in a targeted way
- Concentrate target peptides with as little product loss as possible
- Working with less harsh processing settings that keep the integrity of the peptides
- Use less energy than reverse osmosis systems
- Give designs that can be scaled up and down and are easy to integrate
Among the several advantages of this technology over more conventional separation techniques is the fact that it does not require the use of harsh chemicals, which could otherwise ruin delicate peptide structures. When these technologies are integrated into upstream and downstream processing workflows, you get greater design and improvement flexibility.
Environmental Benefits
By treating water with the same nanofiltration membrane technology, facilities may filter wastewater in an efficient, environmentally friendly, and rule-compliant manner.
Inline Buffer Dilution (ILBD) Systems
Inline buffer dilution technology is a big step forward in the automated preparation and management of buffers. These advanced technologies mix concentrated buffer solutions with Water for Injection (WFI) in real time, giving you exact formulas whenever you need them during the purification process.
Some of the main benefits of ILBD systems are:
Consistent Precision and Reduced Human Error: ILD uses sensors and automated pumps to control pH, conductivity, flow rate, and composition. This reduces variability and manual mistakes, ensuring reproducible formulation across batches—critical for consistency in quality-critical biologics purification.
Space and manpower Efficiency: Gets rid of big buffer storage tanks, which cuts down on the amount of space and manpower needed for the plant.
Seamless Integration: Works with chromatography and filtration equipment to automate all processes.
On-demand, multi-buffer flexibility: ILD allows different buffer formulations to be generated in-line from concentrate, omitting intermediate storage in buffer bags or tanks
Modern ILBD systems are very flexible and powerful. They can quickly process huge batches while keeping fine control over the pH levels and buffer composition. The integration features make it possible to design fully automated workflows that need as little human input as possible and may be repeated as many times as necessary.
DAC Chromatography Columns for Dynamic Axial Compression
Dynamic Axial Compression columns are a huge step forward in the technique of preparative chromatography. These systems keep the packing pressure the same throughout the chromatographic process, which ensures that the media bed properties are the same every time and gets rid of the bed settling and channeling problems that come up with standard packed columns.
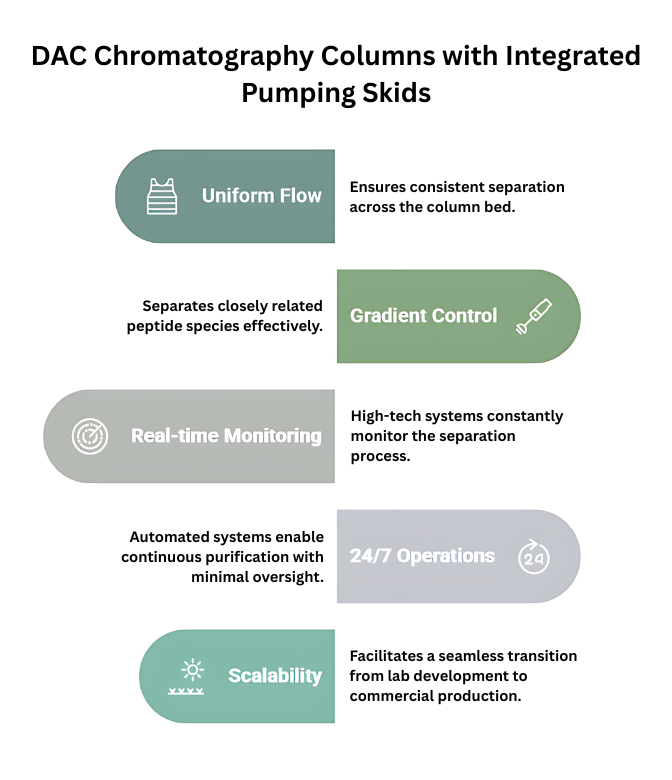
Integrated Pumping Skids
When used with advanced pumping skids, DAC columns give you more control over chromatographic separations than ever before:
- Uniform Flow Distribution: It makes sure that the separation works the same way across the whole column bed.
- Exact Gradient Control: With exact gradient control, it is possible to separate peptide species that are very close to one another.
- Real-time Monitoring: The process is always being watched by high-tech cameras and detection systems.
- 24/7 Operations: Automated systems keep purification going all the time with little supervision.
- Scalability: A smooth move from developing in the lab to full commercial production
The complete pumping skid comes with high-precision pumps, automated valves, modern sensors, detecting systems (UV and refractive index), and complex control software. This fully automated platform can run both isocratic and complicated gradient elution modes. It also follows the criteria for Process Analytical Technology (PAT) for real-time process monitoring and control.
Integrated Workflow Excellence
The combination of these two things makes a streamlined, automated, high-yield purification process:
- Concentration and Desalting: NF membranes only let through target peptides while unwanted compounds are removed.
- Buffer Preparation: ILBD systems make sure that the composition of the buffer is always the same; therefore, there is no need for human preparation.
- Preparative Chromatography: DAC columns with pumping skids keep the best conditions for separation.
This integrated method consistently produces high-purity peptides that satisfy regulatory standards. It also cuts batch cycle times by a lot, cuts down on the amount of space and personnel needed, ensures that the process is GMP-compliant, improves process control, and lowers the danger of contamination.
Sanitech Engineers: Your Technology Partner
Sanitech Engineers makes complete turnkey systems that combine all three technologies. They focus on making precision machines for use in biotechnology.
What We Offer
- Our systems, created by experts, use nanofiltration membranes, ion-exchange chromatography, and advanced separation methods to get the best purifying outcomes.
- We offer fully customized solutions for everything from scaling up in the lab to making products for sale.
- Fully Automated DAC Columns: With built-in pumping skids, packing units, and controls that meet GMP standards
From the moment they are installed, all systems are cGMP-ready. They have digital controls and full validation packages. We promise to provide full site qualification services and global certifications to make sure we follow all the rules.
Clients get shorter processing times, higher yields, and consistently excellent quality that changes the way they do business and makes them more money while still meeting the highest product quality and regulatory standards.
Transform Your Operations Today
Work with Sanitech Engineers to change the way you make peptides by using sophisticated DAC chromatography, integrated nanofiltration, and precise buffer dilution. Get products with improved purity, outcomes that can be repeated, and leaner procedures without the usual complexity.
Get in touch with Sanitech now to start your road toward contemporary, automated, highly efficient production excellence that gives you better outcomes and a competitive edge in today’s tough industry.
FAQs
For gentle peptide processing, nanofiltration is the way to go because it eliminates specific molecules at lower pressures. Reverse osmosis, on the other hand, blocks almost all dissolved solids but needs more energy and can be excessively forceful.
DAC columns keep the same level of media compression during separation, which means they have steady throughput, great repeatability, and easy scaling. They also get rid of settling and channelling problems that are prevalent with regular columns.
Pumping skids let you precisely manage flow rates, pressure gradients, and detection systems. This lets you run your system totally automatically while still meeting regulatory requirements and keeping an eye on performance in real time.
Yes. Sanitech's systems are compliant with cGMP, ASME, PAT, and 21 CFR Part 11 requirements. They come with full validation packages, automated controls with audit trails, and full quality documentation to make sure they are always in line with the rules.